Neuroendocrine tumors (NETs) are a rare form of cancer with just five to six new cases per 100,000 individuals diagnosed each year.* NETs originate in neuroendocrine cells and often develop slowly over several years. Neuroendocrine cells are part of both the endocrine and the nervous system and can be found throughout the body. These cells release neurotransmitters, cell messengers or hormones into the blood, which control how the body works. This means that they play a key role in the interaction between the nervous and the endocrine systems.
NETs frequently originate in the gastroenteropancreatic tract and are referred to as gastroenteropancreatic neuroendocrine tumors (GEP-NETs). GEP-NETs can be found throughout the digestive system or related parts of the body. Although a rare disease, the number of newly diagnosed patients is continuously rising.** GEP-NETs are often asymptomatic and challenging to diagnose. Therefore, they are often diagnosed at a late stage with metastases and with limited possibility of surgical removal. GEP-NETs appear predominantly in patients aged 50-60 years with women being approximately 2.5 times more likely to be affected than men.* In some cases, GEP-NETs can secrete various hormones and are then referred to as functioning GEP-NETs.
Surgery is the main treatment for NETs and often the only course of treatment needed. However, NETs can spread to other parts of the body, or it may not be possible to completely remove the cancer by surgery alone. If this happens, other treatment options such as chemotherapy or targeted therapeutics are available.
Tumor stage and grade play an important role in the treatment of NETs. The stage refers to the state of development of the tumor, i.e. whether the tumor has just emerged, whether it is advanced, or whether it has already spread to other parts in the body (metastasized).
The tumor grade gives information on how quickly the tumor is expected to grow and spread by comparing the abnormal tumor cells with normal, healthy cells under the microscope. In technical terms, this is indicated by the Ki-67 value. Cells of a low-grade tumor (low Ki-67 index), also described as “well-differentiated”, often grow and spread slowly. Cells of a high-grade “poorly differentiated” tumor (high Ki-67 index) grow rapidly.
Current standard therapies for the treatment of GEP-NETs in the pancreas and small bowel include for example somatostatin analogues, chemotherapy, immunotherapy, locoregional treatment (e.g. cytoreduction surgery, radiofrequency ablation [RFA], liver directed intra-arterial intervention), the targeted immunosuppressive therapy everolimus, an mTOR inhibitor that affects the division and growth of cancer cells by blocking the enzyme mTOR, the targeted therapy sunitinib, a multitargeted tyrosine kinase inhibitor or a special form of targeted radiopharmaceutical therapy (RPT) with Lutetium 177 DOTATATE (Lutetium 177 oxodotreotide).*
In addition to standard therapies, new treatment approaches are being investigated in clinical studies to determine if they are safe and effective, such as RPT with n.c.a. Lutetium 177 edotreotide.
* Bryan Oronsky et al., Neoplasia 2017
** Dasari et al., JAMA Oncol 2017
The idea of using radiation therapy as cancer treatment is more than one hundred years old. In 1895, Wilhelm Conrad Röntgen discovered x-rays as a new form of “radiation”, later leading to the development of diagnostic imaging techniques. In 1896, with Henri Becquerel discovering the radiation of natural agents and chemicals and Marie Curie’s fundamental work on radioactivity, the path for the use of radioactive agents as medical treatment options was carved. Soon after, various radiotherapy approaches for a wide range of diseases, often including cancer, were investigated and developed.
How can the power of radiotherapy be used in a precise manner to specifically target the tumor cells?
In contrast to external radiotherapy, where radiation is applied from outside the body, RPT is defined by the intravenous infusion of a radiopharmaceutical, which accurately recognizes tumor cells, into the body. Over the past decades, biomedical investigations of various tumor characteristics and tumor-binding molecules have contributed to the evolution of radiation therapy towards precision oncology and the development of RPT.
Targeted radiopharmaceutical therapy uses radiopharmaceuticals consisting of a medical radioisotope which is used to destroy the tumor cells by emission of a small amount of radiation and a tumor-specific targeting molecule, that binds according to the lock-and-key principle to tumor-specific receptors overexpressed on the tumor cell surface. Combined with a targeting molecule and within a maximum radius of 1.7 mm, the medical radioisotope releases a small amount of radioactivity to the tumor tissue, which is designed to destroy the tumor cells with minimum effect to the surrounding healthy tissue. The highly precise localization can result in the healthy tissue surrounding the targeted tumor being minimally affected. Radiopharmaceuticals are produced in a laboratory under Good Manufacturing Practice (GMP), adhering to strict certified international guidelines.
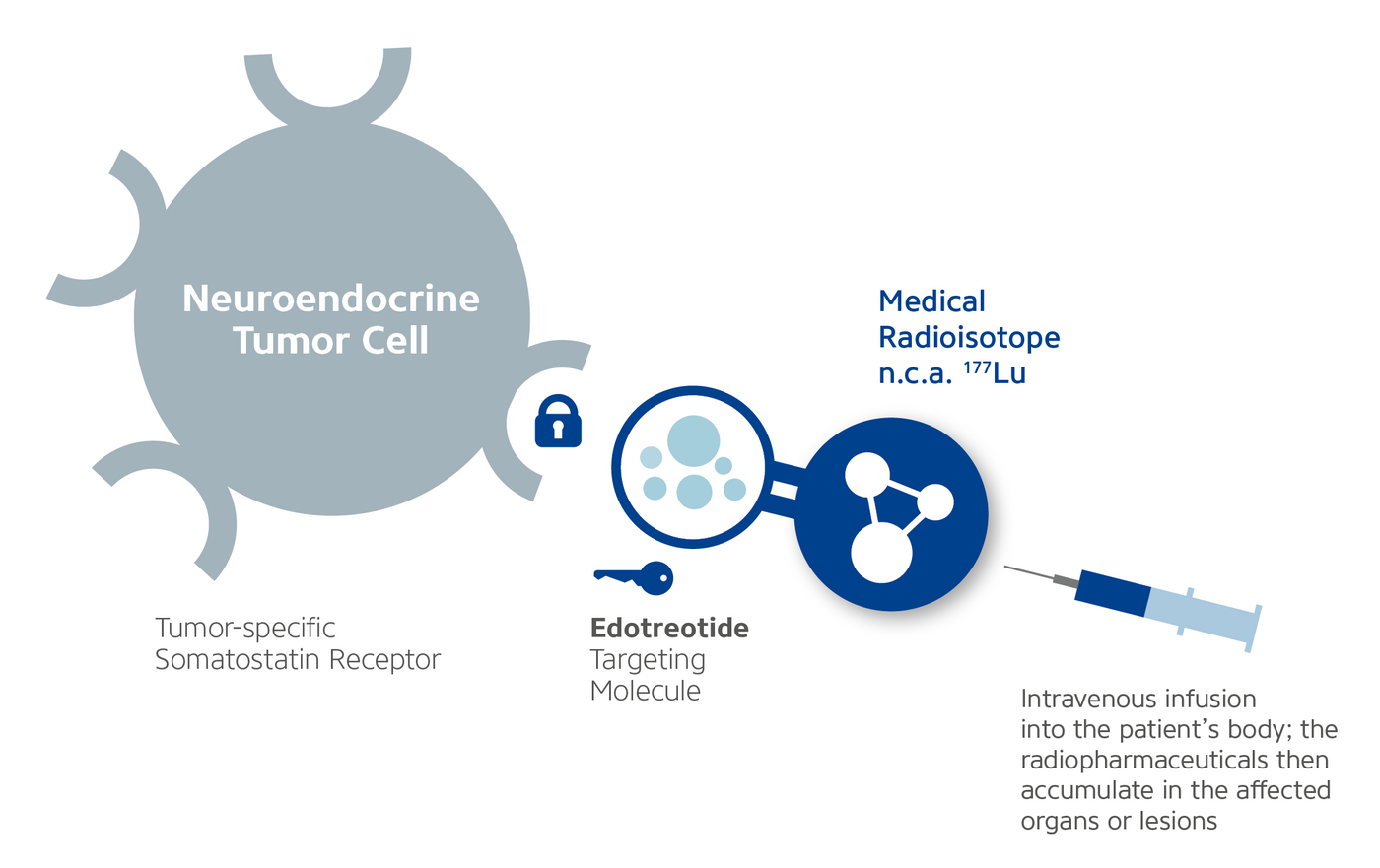
The radiopharmaceutical being investigated in the phase III COMPETE and COMPOSE clinical studies is n.c.a. Lutetium 177 edotreotide. It consists of two components: the medical radioisotope no-carrier-added (n.c.a.) Lutetium-177, which is used to destroy the tumor cells by emission of a small amount of radiation, and the targeting molecule edotreotide, a synthetic form of the peptide hormone somatostatin that targets neuroendocrine tumor-specific receptors.
Healthy neuroendocrine cells have somatostatin receptors on their surface. The neurotransmitter somatostatin regulates the endocrine system within normal body functions by binding to these receptors according to the lock-and-key principle. Targeted radiopharmaceutical therapy uses this same principle to treat NETs. The therapy is based on the fact that neuroendocrine tumor cells often express an increased number of somatostatin receptors on their surface, displaying a unique tumor characteristic that differs from that of healthy neuroendocrine cells.* Just like the naturally produced hormone somatostatin, Eedotreotide binds to these receptors and places the medical radioisotope n.c.a. Lutetium-177 directly onto the diseased neuroendocrine cells so that it accumulates at the tumor site. N.c.a. Lutetium-177 is internalized into the tumor cells and decays, releasing medical radiation (ionizing β-radiation) with a maximum radius of 1.7 mm and destroying the tumor. Thanks to the highly precise localization, the healthy tissue surrounding the targeted tumor is minimally affected.
* Papotti et al., 2002, Virchows Archiv 440(5): 461-75
Please note: n.c.a. lutetium-177-edotreotide is not authorized for marketing in any country at this time.
Please also see trial information on clinicaltrials.gov: COMPETE & COMPOSE
Clinical trials / clinical studies play an important role in the development of new treatment opportunities. Patients can decide to participate in such studies voluntarily. In order to guarantee the safety of participating patients, clinical trials are subject to strict quality control guidelines.
For a clinical trial to be approved by a health authority such as the European National Competent Authorities (NCAs) or the U.S. Food and Drug Administration (FDA), a new therapy has to establish its safety through a preclinical evaluation. This means that the potential treatment is tested for possible dangerous side effects in a controlled manner before it is ever tested in patients. After appropriate information has been provided to the NCAs or FDA and the Ethics Committees, it receives permission to enter clinical testing.
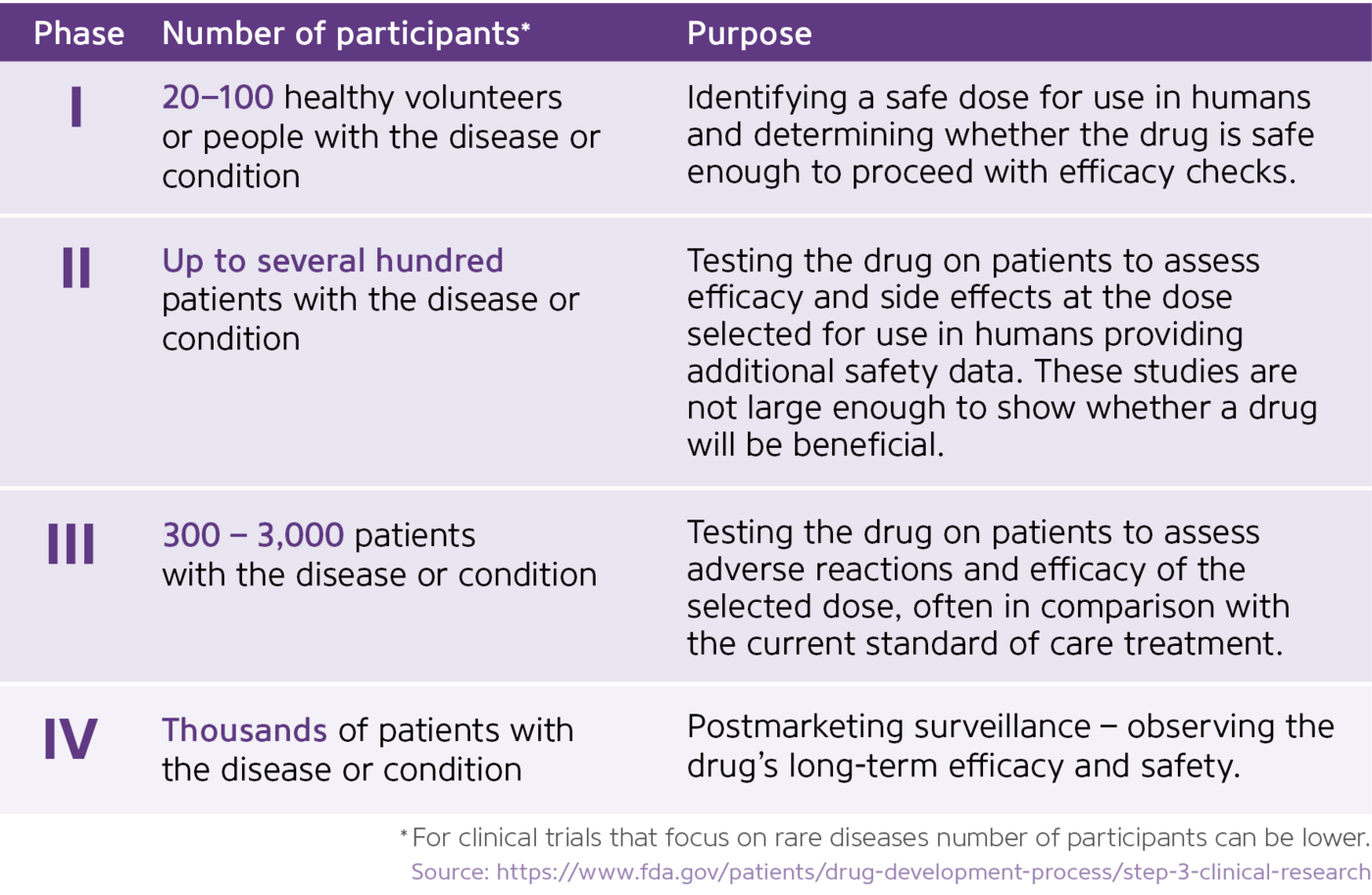
The drug development process normally progresses throughout four phases that investigate and evaluate the efficacy and safety of the substance in an increasing number of patients over the course of many years. If the drug successfully passes through the clinical phases (I, II and III) it is typically approved by the competent regulatory authority for use in routine clinical practice.

Looking for more information? Below you can find additional resources on the background and processes behind clinical studies:
https://www.centerwatch.com/clinical-trials/overview
https://www.clinicaltrials.gov/ct2/about-studies/learn
For further information about ongoing studies please visit the following websites or reach out to your attending physician or a patient organization near you.
https://www.clinicaltrials.gov/
https://www.clinicaltrialsregister.eu/